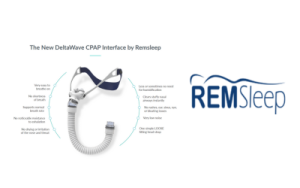
Remsleep Holdings Inc.’s DeltaWave Mask Moves to Substantive Review of FDA 510k Process
Tampa, FL, November 2, 2023 – RemSleep Holdings Inc. (OTCQB: RMSL), a medical device manufacturer dedicated to forever changing the level of treatment provided to Obstructive Sleep Apnea (OSA) patients, is pleased to announce its DeltaWave mask has moved to the Substantive Review portion of the FDA 510k review process. Previously, the company voluntarily withdrew … Continue reading Remsleep Holdings Inc.’s DeltaWave Mask Moves to Substantive Review of FDA 510k Process
0 Comments