
POMPANO BEACH, Fla., January 13, 2025 – PRISM MediaWire – BioStem Technologies, Inc. (OTC: BSEM) (“BioStem”, “we”, “us”, or “our”), a leading MedTech company focused on the development, manufacturing, and commercialization of placental-derived products for advanced wound care, today announced selected preliminary financial results for the fiscal fourth quarter and full year ended December 31, 2024.
2024 Preliminary Fourth Quarter and Year End Results:
- Fourth quarter net revenue is expected to be approximately $102.9 million, which represents a 794% increase over the fourth quarter of 2023, driven by solid performance across the product portfolio.
- Full-year 2024 net revenue is expected to be approximately $301.8 million, up from $16.7 million for fiscal year 2023, reflecting an increase of $285.1 million, or 1,702%.
- Fourth quarter gross profit is expected to be approximately $99.3 million, compared to $10.9 million for the fourth quarter of 2023, which represents an 811% increase.
- Full-year 2024 gross profit is expected to be approximately $288.1 million, compared to $15.4 million for fiscal year 2023, which represents an 1,775% increase.
- This exceptional year-over-year growth reflects the robust adoption of AmnioWrap2 OneView™ in the post-acute care market and the successful introduction of Vendaje AC®, supported by the targeted commercial strategies of Venture Medical, LLC (“Venture”), BioStem’s exclusive sales and marketing partner. Leveraging the advanced analytics and market insights provided by the Venture OneView™ platform, BioStem has effectively expanded its market presence, driving accelerated product adoption and strengthening its position as a leader in advanced wound care solutions.
“2024 has been an extraordinary and transformative year for BioStem, defined by record-breaking growth and a series of groundbreaking milestones that underscore our leadership in advanced wound care,” said Jason Matuszewski, Chief Executive Officer of BioStem. “Achieving over 1,700% year-over-year revenue growth is a testament to the relentless execution of our strategic initiatives, the innovation behind our products like AmnioWrap2™ and the newly-launched Vendaje AC®, and the dedication of our team.
Our success has been further catalyzed by our valued partnership with Venture and their state-of-the-art Venture OneView™ platform, which has revolutionized our distribution capabilities, enhanced visibility within the market, and accelerated product adoption. This synergy exemplifies the power of collaboration in driving impactful results.
As we look to 2025, I am energized by the opportunities ahead, particularly the anticipated finalization of the LOI with ProgenaCare, a key step in broadening our product portfolio and deepening our impact across the continuum of wound care. Coupled with our investments in R&D, clinical trials, and the expansion of our world-class team, we believe this acquisition positions us to continue delivering cutting-edge solutions that improve patient outcomes and create lasting value for all stakeholders.
We are deeply grateful for the unwavering support of our shareholders, customers, and partners, and we remain committed to leveraging innovation, collaboration, and excellence to shape the future of wound care and beyond.”
About BioREtain®:
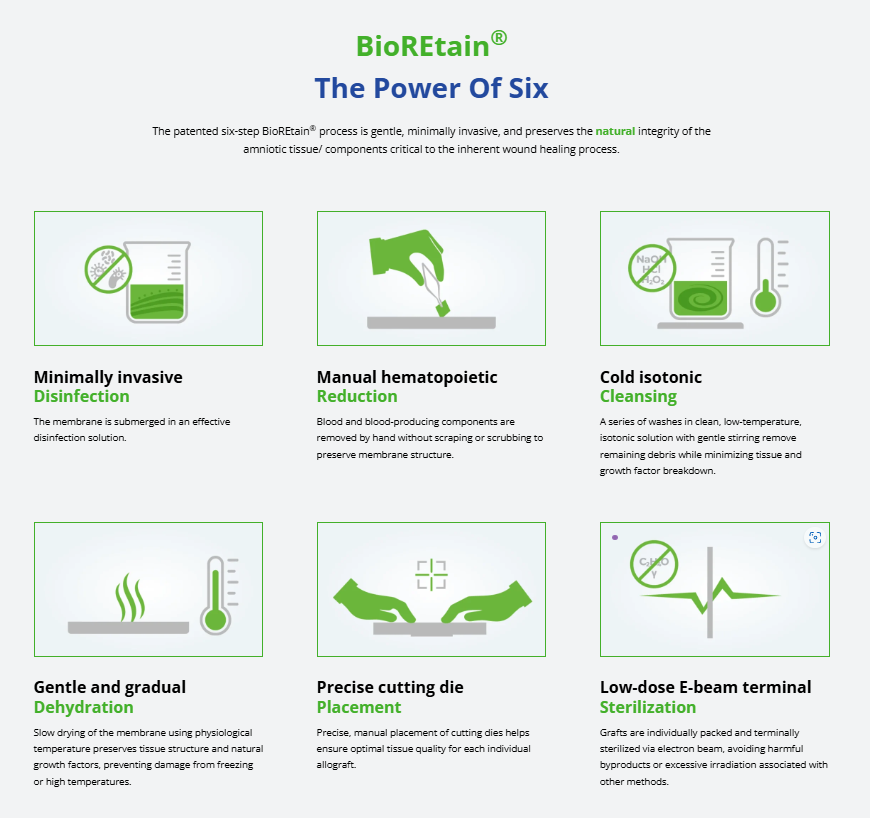
BioStem’s placental allografts are processed utilizing BioStem’s proprietary BioREtain®method, which preserves the tissue’s endogenous biological properties while maintaining the structure and matrix found in fresh perinatal tissue. The patented six-step BioREtain®process is gentle, minimally invasive, and preserves the natural integrity of the amniotic tissue/components critical to the wound treatment process. For a full overview of BioREtain, please visit: https://biostemtechnologies.com/our-science/#six-steps.
Join BioStem’s Distribution List & Social Media:
To follow the latest developments at BioStem, sign-up to BioStem’s email distribution list HERE, and follow us on Twitter and LinkedIn.
About BioStem Technologies, Inc. (OTC: BSEM):
BioStem Technologies is a leading innovator focused on harnessing the natural properties of perinatal tissue in the development, manufacture, and commercialization of allografts. BioStem is focused on manufacturing products that change lives, leveraging its proprietary BioREtain® processing method. BioREtain® has been developed by applying the latest research in advanced wound care, focused on maintaining growth factors and preserving tissue structure. BioStem Technologies’ quality management system and standard operating procedures have been reviewed and accredited by the American Association of Tissue Banks (“AATB”). These systems and procedures are established per current Good Tissue Practices (“cGTP”) and current Good Manufacturing Processes (“cGMP”). Our portfolio of quality brands includes AmnioWrap2™, VENDAJE®, VENDAJE AC®, and VENDAJE OPTIC®. Each BioStem Technologies placental allograft is processed at BioStem’s FDA registered and AATB accredited site in Pompano Beach, Florida. For more information visit biostemtechnologies.com and follow us on Twitter and LinkedIn.
Forward-Looking Statements:
Except for statements of historical fact, this release also contains forward-looking statements within the meaning of applicable securities laws. These forward-looking statements relate to expectations or forecasts of future events. Forward-looking statements may be identified using words such as “forecast,” “intend,” “seek,” “target,” “anticipate,” “believe,” “expect,” “estimate,” “plan,” “outlook,” and “project” and other similar expressions that predict or indicate future events or trends or that are not statements of historical matters. Forward-looking statements in this release include statements regarding BioStem’s plans and expectations for future performance, including: (i) BioStem’s expectations about projected net revenue and gross profit for the quarter and year end; (ii) BioStem’s expectations regarding product adoption and market presence; (iii) BioStem’s strategic initiatives as well as its ability to accelerate growth in 2025 and beyond; (iv) BioStem’s expectations regarding its timing and ability to close the Progenacare acquisition pursuant to the terms of any agreement entered into by and between the parties, as well as any impact that such acquisition will have on BioStem’s business and operations and (v) BioStem’s ongoing commitment to leverage innovation and collaboration to deliver cutting-edge solutions that improve patient outcomes and create lasting value for stakeholders. The actual financial and operating results that will be reflected in its audited financial statements, when they are completed and publicly disclosed, may differ from these preliminary results.
Forward-looking statements with respect to the operations of BioStem, strategies, prospects and other aspects of the business of BioStem are based on current expectations that are subject to known and unknown risks and uncertainties, which could cause actual results or outcomes to differ materially from expectations expressed or implied by such forward-looking statements. The following is a list of factors, among others, that could cause actual results to differ materially from those contemplated by the forward-looking statements: the impact of any changes to the reimbursement levels for BioStem’s products; the significant and continuing competition that BioStem faces, which could adversely affect its business, results of operations and financial condition; rapid technological change that could cause BioStem’s products to become obsolete, and the risk that if BioStem does not enhance its product offerings through its research and development efforts, it may be unable to effectively compete; BioStem’s ability to convince physicians that its products are safe and effective alternatives to existing treatments and that its products should be used in their procedures; BioStem’s ability to raise funds to expand its business; the risk that BioStem may incur significant losses in the future; changes in applicable laws or regulations; the possibility that BioStem may be adversely affected by other economic, business, and/or competitive factors; BioStem’s ability to maintain production of its products in sufficient quantities to meet demand; and the Company’s ability to consummate an agreement with Progenacare. You should carefully consider the foregoing factors and the other risks and uncertainties described in the “Risk Factors” section of the Registration Statement on Form 10, and any subsequent amendments, initially filed with the Securities and Exchange Commission (“SEC”) on September 27, 2024, and in any subsequent filings with the SEC.
You are cautioned not to place undue reliance upon any forward-looking statements, which speak only as of the date made. Although it may voluntarily do so from time to time, BioStem undertakes no commitment to update or revise the forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable securities laws.
BioStem Technologies, Inc.
Phone: 954-380-8342
Website: http://www.biostemtechnologies.com
E-Mail: info@biostemtech.com
Twitter: @BSEM_Tech
Facebook: BioStemTechnologies
PCG Advisory
Jeff Ramson
jramson@pcgadvisory.com
646-863-6893
Source: BioStem Technologies, Inc.


