
Tampa, FL, October 6, 2023 – RemSleep Holdings Inc. (OTCQB: RMSL), a medical device manufacturer dedicated to forever changing the level of treatment provided to Obstructive Sleep Apnea (OSA) patients, announces it has submitted the DeltaWave mask to the FDA for 510K review.

After a series of unfortunate and frustrating delays due to Covid, industry recalls, and 3rd party testing that required retesting certain tests, RemSleep is excited to announce it has submitted the DeltaWave mask for FDA 510K Review. The company invites investors to review the submission process timeline by the FDA at the following link: https://www.fda.gov/medical-devices/premarket-notification-510k/510k-submission-process#timeline. A summary graphic of the review process is included below:
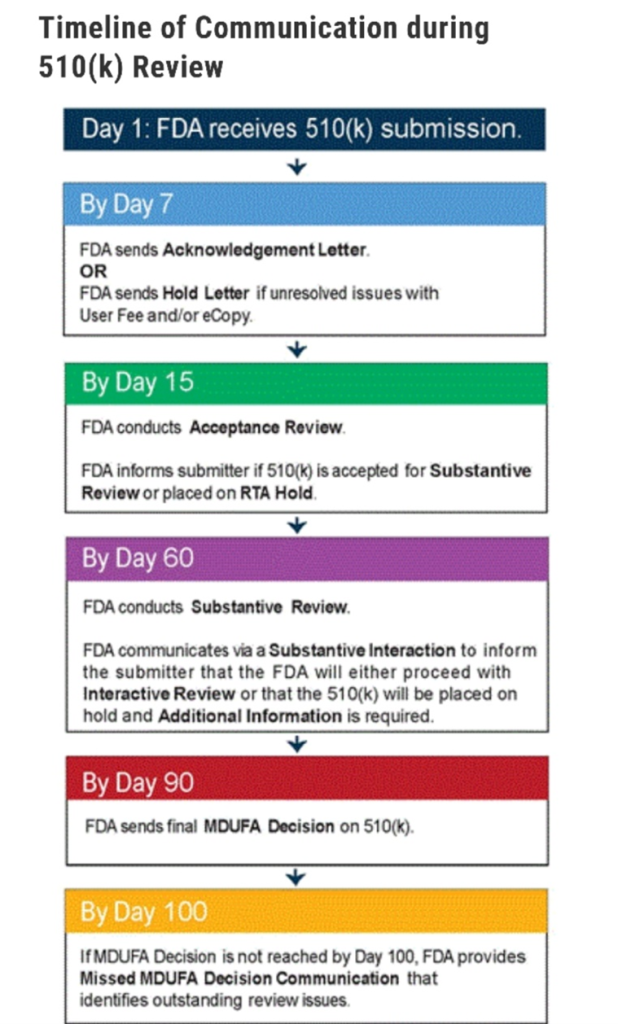
RemSleep CEO, Tom Wood, commented: “It is with excitement and relief that we can finally share this with our investors and definitively move forward with the DeltaWave. As we’ve said before, we have certainly had our share of setbacks but, throughout the process, we have been compelled by our confidence in the DeltaWave both in its safety, and its superior performance relative to traditional masks on the market. Further, with the guidance and expertise from our Senior Director for Regulatory Compliance, Judy Strzepek, we are confident we have provided all and any information the FDA will need for its substantive review. While we cannot anticipate every area the FDA might have further questions, her past regulatory experience was pertinent in designing the testing protocol for the DeltaWave to help mitigate the need for additional information requests. As always, we thank our investors for their patience and confidence in RemSleep.”
During the review process, the company will continue to focus on the business objectives outlined previously in its shareholder letter including: aligning with distributors and partners for post clearance sales and marketing, CPAP and related equipment sales and marketing in conjunction with the DeltaWave, and continued development of the next generation DeltaWave masks.
RemSleep will continue to update investors as information becomes available and will be confirmed on the company website: www.remsleep.com; and through the company Twitter feed: @RemsleepInc.
About RemSleep Holdings Inc.
RemSleep Holdings Inc. is a medical device manufacturer dedicated to forever changing the level of treatment provided to obstructive Sleep Apnea patients. Our focus is primarily designing and manufacturing devices and products for the treatment of Sleep Apnea and other respiratory conditions. With over 30 years of collective experience in CPAP therapy, the RemSleep team has extensive knowledge and understanding of CPAP and the challenges of patient compliance. We diligently strive for our products to make the difference and improve the condition of those suffering from Sleep Apnea. www.remsleep.com
https://twitter.com/RemsleepInc
Forward-Looking Statements
Some statements in this release may contain forward-looking information. All statements, other than of historical fact, that address activities, events or developments that the Company believes, expects or anticipates will or may occur in the future (including, without limitation, statements regarding potential acquisitions and financings) are forward-looking statements. Forward-looking statements are generally identifiable by use of the words “may”, “will”, “should”, “continue”, “expect”, “anticipate”, “estimate”, “believe”, “intend”, “plan” or “project” or the negative of these words or other variations on these words or comparable terminology. Forward-looking statements are subject to a number of risks and uncertainties, many of which are beyond the Company’s ability to control or predict, that may cause the actual results of the Company to differ materially from those discussed in the forward-looking statements. Factors that could cause actual results or events to differ materially from current expectations include, among other things, without limitation, the inability of the Company to obtain sufficient financing to execute the Company’s business plan; competition; regulation and anticipated and unanticipated costs and delays, and other risks disclosed in the Company’s public disclosure record on file with the relevant securities regulatory authorities. No information in this press release should be construed as any indication whatsoever of the Company’s future revenues, results of operations or stock price. Although the Company has attempted to identify important factors that could cause actual results or events to differ materially from those described in forward-looking statements, there may be other factors that cause results or events not to be as anticipated, estimated or intended. Readers should not place undue reliance on forward-looking statements. The forward-looking statements included in this news release are made as of the date of this news release and the Company does not undertake an obligation to publicly update such forward-looking statements to reflect new information, subsequent events or otherwise unless required by applicable securities legislation.
Investor Relations Contact:
Preya Narain
info@preya.co
SOURCE: RemSleep Holdings Inc



